- info@carbocraft.co.za
- 011 234 0675
- MONDAY-THURSDAY 8:30-4:30 FRIDAYS 8:30-3:30
Molecular Sieves (MS) & Zeolite
ZEOCHEM, our esteemed supplier of molecular sieves, chromatography silica gels, and deuterated compounds, is world renowned for exceptionally high-quality products and global support services!
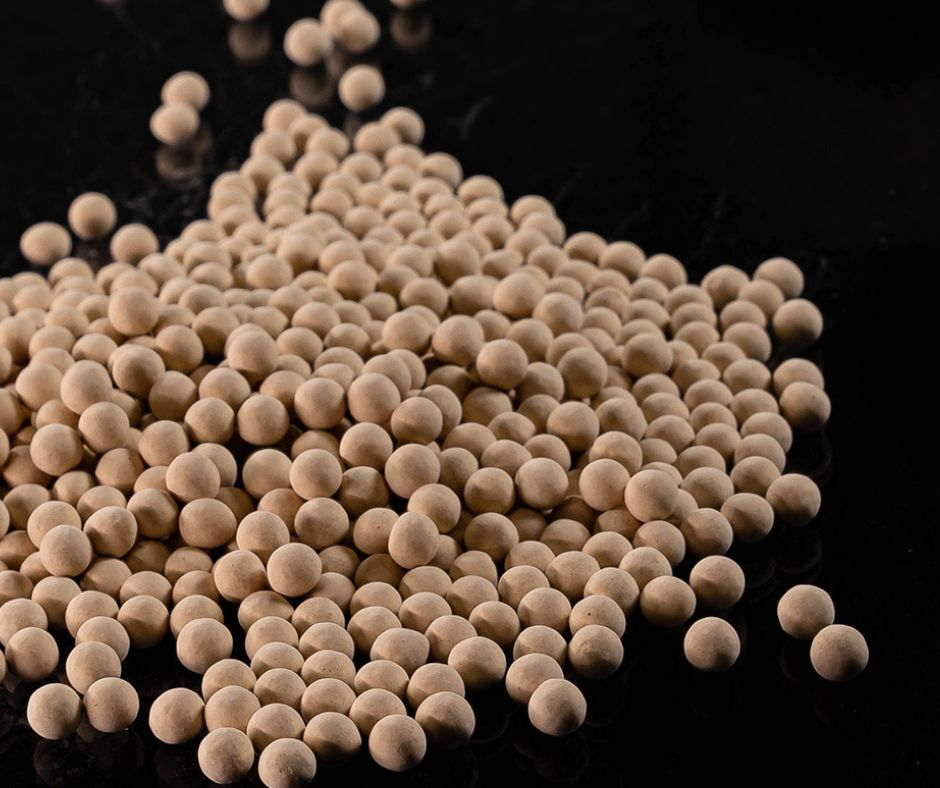
Molecular sieves are solid materials with highly regular nano-porous structures, capable of selectively adsorbing molecules based on their size, shape, and polarity. They are commonly composed of metal or metal oxide frameworks, such as zeolites or aluminosilicates. The pore size can be tailored for specific applications, enabling their use in vast separation and purification processes.
Primary applications of molecular sieves include:
- Adsorption of volatile organic compounds (VOC): Molecular sieves can selectively adsorb VOCs from air or gas streams, aiding in air purification and odour control.
- Catalyst support: Molecular sieves can serve as a host matrix for catalysts by providing high surface area and shape-selective properties.
- Dehydration: Molecular sieves are used to remove water and moisture from gases or liquids, making them valuable in drying processes for gases such as natural gas, air compression, or refrigerant systems.
- Desiccants: Molecular sieves are employed in various applications where moisture control is crucial, such as electronics manufacturing, pharmaceuticals, or preservation of sensitive materials.
- Gas separation: Molecular sieves are employed in gas separation processes, such as the removal of carbon dioxide from natural gas, nitrogen enrichment from air, or the separation of specific hydrocarbon isomers.
- Petrochemical refining: Molecular sieves are utilized in the purification of feedstocks, cracking processes, and separation of complex hydrocarbon mixtures.
- Oxygen enrichment: Molecular sieves are used in medical oxygen concentrators or other systems requiring high-purity oxygen.
Zeolites, in close relation to molecular sieves, are by technical definition, a type of crystalline aluminosilicate mineral, primarily composed of aluminium, silicon, and oxygen atoms arranged in a three-dimensional framework. They have a porous structure with regularly spaced channels and cavities that enable the selective adsorption and exchange of ions and molecules.


MASS TRANSFER – WHAT IS IT?
Mass transfer refers to the transfer of mass (atoms, molecules, or particles) from one phase to another, such as from a gas to a liquid or vice versa. It is driven by concentration gradients or differences in chemical potential between the phases. The process of mass transfer involves diffusion, convection, or a combination of both. Molecular sieves play a crucial role in mass transfer due to their unique structure and adsorption properties. The nano-porous structure of molecular sieves provides a large surface area, allowing for enhanced interaction with the molecules present in the fluid mixture.
When a fluid mixture encounters a molecular sieve, molecules that fit into the pores of the sieve can be selectively adsorbed onto its surface. This adsorption process occurs based on the size, shape, and polarity of the molecules. Larger molecules that are too big to fit into the pores will not be adsorbed, while smaller molecules that match the pore size will be preferentially adsorbed. By selectively adsorbing certain molecules, molecular sieves facilitate the separation and purification of fluid mixtures. They allow the targeted removal of unwanted components, such as moisture, impurities, or specific gas molecules, from a mixture. This process occurs as the fluid mixture diffuses through the pores of the sieve, and the molecules of interest are adsorbed, while the others pass through.
Molecular sieves can also be used in processes involving gas-phase reactions. By selectively adsorbing certain reactant molecules onto their surface, they can help control the reaction kinetics and increase the yield or selectivity of desired products. The mass transfer capability of molecular sieves is primarily due to their porous structure, large surface area, and selective adsorption properties. These characteristics enable molecular sieves to play a vital role in various applications involving separation, purification, catalysis, and more, where the transfer of mass between different phases is crucial.
CARBOCRAFT is proud to have partnered up with ZEOCHEM, a manufacturer of high-quality molecular sieves and chromatography gels, established more than 190 years ago, with headquarters in Switzerland.
ZEOCHEM molecular sieve adsorbents are highly efficient crystalline aluminosilicates. During production, their unique structure allows the water of crystallization to be removed in a very specific way, leaving a highly porous crystalline structure behind. These pores or “cages” have a high affinity to re-adsorb water or other polar molecules. Aided by strong ionic forces or electrostatic fields brought on by the presence of cations such as sodium, calcium, and potassium, and due to the remarkably high internal surface area of around 1,000 m2/g, ZEOCHEM molecular sieves can adsorb a considerable amount of water or other compounds. If the fluid to be adsorbed is a polar compound, it can be adsorbed with a very high loading capacity, even at very low concentrations of the contaminants. ZEOCHEM molecular sieves can therefore adsorb or remove or separate many gas or liquid impurities, to very low levels (ppm or less). Another feature of our ZEOCHEM molecular sieve adsorbents is the high efficacy in which they separate gases or liquids by molecular size or polarity. The pore or “cage” openings are of the same size as a range of different molecules e.g. in the case of hydrocarbon paraffins, the normal, straight-chained molecules can fit into the pores and be adsorbed and separated, while the branched molecules cannot, and thus pass through the molecular sieve bed un-adsorbed.


MOLECULAR SIEVE TYPES
ZEOCHEM manufactures a wide range of zeolites used as molecular sieve (MS) adsorbents, covering the full spectrum of typical adsorption applications. We have the right type of molecular sieve for your process.
Here is an overview of our primary grades of MS products that we supply, with the main applications that we serve:
Refers to a specific variation of zeolite with a pore size that allows the selective adsorption of molecules up to a certain size. The “3A” designation corresponds to the approximate diameter of the pore openings, which is about 3 Angstroms (or 0.3 nanometers).
- Zeolite 3A is composed of an alumina-silicate framework with three-dimensional channel structures. The framework consists of interconnected tetrahedra of aluminium, silicon, and oxygen atoms, giving rise to a porous structure with regularly arranged channels and cavities.
- The 3A structure is ideal for adsorbing and selectively capturing molecules, particularly those with a diameter of three Angstroms or smaller. It exhibits a high affinity for adsorbing small polar molecules, such as water, carbon dioxide, methanol, ethanol and reactive monomers such as olefins.
- One prominent application of zeolite 3A is dehydration, where it is employed to remove water or water vapor from gases and liquids. Due to its affinity for water molecules and its ability to selectively adsorb them, it is widely used in drying processes for gases like natural gas, air compressors, and refrigerants.
- Additionally, zeolite 3A finds applications in separating small polar molecules from mixtures, such as the removal of carbon dioxide from natural gas or air purification. Its ability to selectively adsorb small polar molecules makes it valuable in various industrial processes requiring purification, separation, or selective adsorption based on molecule size and polarity.
- Type 3A is produced by ion-exchanging potassium onto Type 4A in place of sodium.
Refers to a specific variant of zeolite characterized by its specific pore size and adsorption properties. The “4A” designation indicates that the zeolite has a pore opening of approximately 4 Angstroms (or 0.4 nanometers).
- Zeolite 4A possesses a crystalline structure composed of interconnected silica (SiO2), alumina (Al2O3), and oxygen (O) atoms. It features a three-dimensional framework of tetrahedral units, forming channels and cavities within its structure.
- The 4A zeolite structure is highly useful for selective adsorption, particularly for molecules and ions with diameters smaller than 4 Angstroms. It exhibits a strong affinity for adsorbing and exchanging calcium and sodium ions. The primary application of zeolite 4A is in water softening and ion exchange processes. It can effectively remove hardness-causing minerals, such as calcium and magnesium ions, from water. By exchanging these ions with more desirable sodium ions, zeolite 4A helps reduce the buildup of scale.
- Zeolite 4A finds applications in drying various gases and liquids. It has a high affinity for adsorbing water molecules, making it useful for removing moisture from air or gases in processes like air compression, refrigeration, and natural gas treatment. Its ability to selectively adsorb water while allowing other components to pass through is a valuable feature in these applications.
Refers to a specific variation of zeolite, a porous mineral crystal made of aluminium, silicon, and oxygen. It belongs to the larger family of zeolites, which are known for their unique structures and ability to act as molecular sieves. Zeolite 5A has a specific pore size of approximately 5 Angstroms (0.5 nanometers) and exhibits high selectivity for molecules with a kinetic diameter of less than 5A, such as carbon dioxide, water, and smaller hydrocarbons.
- The specific pore structure and size of zeolite 5A make it especially suitable for applications such as gas separation, dehydration of gases and liquids, and the removal of impurities and contaminants from feed streams. Its adsorption and molecular sieving properties make zeolite type 5A a valuable material in industries such as petrochemicals, air separation, and natural gas processing.
- Commonly referred to as the calcium-exchanged form of the Type A zeolite. The strong ionic forces of the divalent calcium cation make 5A an excellent choice for removing carbon dioxide, carbon monoxide, hydrogen sulphide and other weakly polar molecules. This product is also effective for the bulk separation of normal and iso-paraffin hydrocarbons.
Refers to another variant of zeolite, characterized by its unique structure and pore size. Like other zeolites, it is composed of aluminum, silicon, and oxygen atoms arranged in a crystalline lattice. The “13X” in the name refers to the approximate pore size of 13 Angstroms (1.3 nanometers).
- With its relatively larger pore size compared to zeolite 5A, zeolite 13X is known for its excellent adsorption capacity for larger molecules and contaminants. It is commonly used in various industrial applications due to its ability to selectively adsorb molecules with a kinetic diameter of less than 13 angstroms. This makes it particularly effective in gas separation processes and the removal of impurities or contaminants in the petrochemical industry.
- Zeolite 13X is also widely used for the purification and dehydration of gases, particularly in the natural gas industry. Additionally, it finds applications in air separation, oxygen generation, the removal of sulphur compounds from various gas streams, and the drying of solvents and liquids.
The sodium form of zeolite X has a much larger pore opening than the Type A crystals. It also has the highest theoretical capacity of the common adsorbents and very good mass transfer rates. Type 13X removes impurities too large to fit into the Type A zeolites and is also often used to separate nitrogen from air to produce a high purity oxygen stream.
Zeolite Type Y is a specific variant of zeolite that belongs to the family of zeolites known as Faujasites. It is named after the French crystallographer Barthélemy Faujas de Saint-Fond, who first described these minerals. Zeolite Y has a specific crystalline structure consisting of aluminium, silicon, and oxygen atoms that form a three-dimensional framework. One of the distinctive features of zeolite Type Y is its large pore size, with a diameter typically ranging from 12 to 13 Angstroms (1.2 to 1.3 nanometers).
- These larger pores make zeolite Y highly effective in adsorbing and separating larger molecules in various industrial processes. It exhibits excellent thermal and chemical stability, which allows it to withstand rigorous conditions during catalytic reactions.
- Zeolite Y is widely used as a catalyst and adsorbent in the petroleum and petrochemical industries. It is commonly employed in processes such as catalytic cracking, isomerization, hydrocracking, and the removal of nitrogen and sulphur compounds from petroleum feedstocks. Its large pore size enables the accommodation of bulky molecules, making it especially useful in catalyzing reactions involving larger hydrocarbons.
- Similar in structure to the Type X zeolite, Type Y has a higher silica to alumina ratio, offering some improved adsorption of hydrophobic compounds and imparting some acid resistance.
Pentasil zeolites are characterized by having a unique chemical structure composed of silicon, aluminum, and oxygen atoms arranged in a three-dimensional framework. The term “pentasil” highlights their five-membered rings within the zeolite structure.
- Pentasil zeolites are highly porous materials with well-defined channels and cavities of uniform size, allowing for selective adsorption and ion-exchange properties. The pentasil structure gives these zeolites specific catalytic properties and shape-selective capabilities.
- These zeolites exhibit exceptional thermal and hydrothermal stability, making them suitable for a wide range of applications in the chemical, petrochemical, and refining industries. They are often used as catalysts or catalyst supports due to their shape-selective nature, high surface area, and tailored pore structures, which enable efficient molecular diffusion and optimal reactant-product selectivity.
- Common examples of pentasil zeolites include zeolite Beta, zeolite ZSM-5 (Zeolite Socony Mobil-5), and zeolite Omega. These materials have diverse applications, including chemical synthesis, petroleum refining, environmental remediation, gas separation, and more, where their unique structure and properties allow for precise control and enhancement of various chemical reactions and processes.
Zeolite type mordenite is a specific variant of zeolite known for its characteristic pore structure and high thermal stability. It is named after the town of Morden in Canada, where it was first discovered. Mordenite is classified as a fibrous zeolite, as its crystal structure forms elongated and thin needle-like structures. The mordenite structure is made up of aluminium, silicon, and oxygen atoms arranged in a three-dimensional framework. It exhibits channel-like pores and interconnected channels, which allow for the adsorption and diffusion of molecules within its structure. The pore size of mordenite typically ranges from 5 to 7 Angstroms (0.5 to 0.7 nanometers).
- One of the notable properties of mordenite is its high thermal stability, which allows it to withstand elevated temperatures without significant structural degradation. Because of this property, mordenite finds applications in catalytic reactions that require high-temperature conditions, such as the conversion of alcohols or the synthesis of various chemicals.
- Mordenite is commonly used as a catalyst and adsorbent in the petrochemical and chemical industries. It is utilized in processes such as isomerization, hydrocracking, selective adsorption, molecular sieving, and the removal of nitrogen compounds from gas streams. Its pore size and structure enable it to selectively adsorb smaller molecules while excluding larger ones.
- Zeolite type mordenite plays a crucial role in various industrial applications, especially in catalysis and adsorption processes requiring high-temperature stability and selective molecular sieving capabilities. Mordenite is also highly acid-resistant, making it a suitable catalyst carrier.
ZEOCHEM MOLECULAR SIEVES
- Dehydration of natural gas and natural gas liquids such as propane and butane
- Removal of CO2, H2S, mercaptans and other impurities from natural gas or natural gas liquids
- Dehydration and CO2 removal from air for cryogenic processing
- Separation of high-purity oxygen from air via Pressure Swing Adsorption (PSA) or Vacuum Swing Adsorption (VSA)
- Recovery of inert gases by adsorption
- Drying and purification of H2 using either Thermal Swing or Pressure Swing Adsorption (PSA)
- Purification of natural gas feed
- Drying and purification of the reformer gas prior to the ammonia loop
- Treating of ammonia off-gas streams
- Olefin and polyolefin production
- Drying of cracked olefin gas, ethylene, and propylene products
- Dehydration and purification of ethane feed to the cracker
- Impurity removal from polyolefin plant feed (catalyst protection from oxygenates, sulfides)
- Dehydration of light-ends (e.g. olefin) for cryogenic recovery
- Purification of feed to catalytic processes such as isomerization, dimerization, and alkylation units
- Drying of solvents
- Purification of feed streams to catalytic processes such as ethyl benzene
- Underground cavern drying and treating
- Storage tank breathers
- Product recovery from vent streams
- Bulk water removal at the azeotrope
- Purification of food- and pharmaceutical-grade ethanol
INDUSTRIAL APPLICATIONS
- Prevention of condensation in the insulating glass space of windows
- Dehydration and trace contamination removal in enclosed spaces
- Removal of odours
- Containment and concentration of solvent vapours
- Removal of water of reaction in urethane formulations
- Cell size control in blown foams
- Plastic pellet driers prior to blow- or injection-moulding
- Dehydration and filtration of refrigerant loops
- Dehydration and purification of compressed breathing air
- Dehydration of compressed air for pneumatic tools
- Truck and railroad air brake driers
- Oxygen concentrators for respiratory therapy
ZEOCHEM LIQUID CHROMATOGRAPHY
Chromatography Gels: Liquid chromatography is one of the most widely used purification methods in the pharmaceutical and natural extract industry. This extremely efficient separation runs under low stress conditions, thus ensuring a high yield of the target compound which is adsorbed on the chromatographic material together with impurities and by-products.
The chromatographic material (stationary phase) is packed in a chromatographic column and the mixture to be purified travels through the column suspended in a solvent mobile phase. By selecting the correct composition of the mobile phase, the adsorption of the target compound and of the by-products can be influenced, which allows their separation. Silica gel is the stationary phase of choice in liquid chromatography. It is an amorphous porous material, derived from silicon dioxide by polycondensation of water glass and an acid. It has a very high surface area consisting of OH-groups ready to interact with polar compounds. Depending on the production method, two different kinds of silica gel are available.
- The irregular silica gel is widely used in flash and thin layer chromatography and is regarded as the base line product, whereas spherical silica gel allows a high-end stationary phase, giving better performance, higher process robustness and significantly less product contamination. It can be cleaned and repacked with “cleaning in place” methods while the standard irregular silica gel will normally be disposed of after use.
- Both materials can be modified on the surface to open many other chromatographic modes, such as reversed-phase chromatography (non-polar compounds), supercritical fluid chromatography and special normal-phase chromatography.
- ZEOCHEM supplies a full line of irregular and spherical chromatography gels, covering the full spectrum of liquid chromatography applications e.g. Amines, API, Carbohydrates, Insulin, Lipids, Natural Products, Peptides, Small Molecules, Small Proteins, Steroids, Vitamins, etc.