- info@carbocraft.co.za
- 011 234 0675
- MONDAY-THURSDAY 8:30-4:30 FRIDAYS 8:30-3:30
Propylene Glycol (PG) & Polyethylene Glycol (PEG)
CARBOCRAFT supplies a diverse range of glycol derivatives, chief among them being Propylene Glycol (PG) & Polyethylene Glycol (PEG)! Glycol derivatives play an important role in various industries today due to their diverse range of unique properties and specific functionalities. Here are some key reasons why they are significant:

Antifreeze and De-icing Agents: Ethylene glycol derivatives are widely used as antifreeze and de-icing agents in automotive and aircraft industries. These derivatives lower the freezing point of water, preventing engine damage or equipment failure in cold climates.
Chemical Intermediates: Glycol derivatives are often used as intermediates in the production of various chemical compounds. For example, ethylene glycol is a key intermediate in the synthesis of polyester fibres, polyethylene terephthalate (PET) resins used in plastic bottles, and numerous other polymers.
Heat Transfer Fluids: Glycol derivatives, specifically ethylene glycol, are commonly used as heat transfer fluids in numerous applications, including HVAC systems, industrial cooling, and automotive engines. They have excellent thermal conductivity and stability, helping to maintain temperature control and prevent freezing or overheating.

Humectants and Moisturizers: Some glycol derivatives, like propylene glycol, exhibit humectant properties, meaning they can attract and retain moisture. These derivatives are used extensively in the personal care and cosmetic industries for their moisturizing effects in products such as lotions, creams, and shampoos.
Pharmaceuticals and Medical Applications: Some glycol derivatives are used in pharmaceutical applications, either as active ingredients or as excipients in drug formulations. They can enhance solubility, stability, and bioavailability of drugs. Additionally, certain derivatives are utilized in medical applications such as pharmaceutical injections, topical creams, and oral formulations.
Solvent Properties: Many glycol derivatives serve as effective solvents for a wide range of substances. They can dissolve and stabilize both polar and non-polar compounds, making them valuable in industries such as paints, coatings, adhesives, and cleaning products.
Propylene Glycol (PG) is a chemical compound with the formula C3H8O2. It is a viscous, colourless liquid with a slightly sweet taste. It is classified as a diol, meaning it contains two hydroxyl groups (-OH) attached to a three-carbon chain.
The most common production process of propylene glycol involves the hydrogenolysis of propylene oxide using hydrogen gas and a catalyst, typically a metal oxide or a sulphide. This reaction leads to the formation of propylene glycol and water. Key applications for PG include:
- Antifreeze and Coolant: Propylene glycol is commonly used as a non-toxic antifreeze in automotive and HVAC systems. It helps prevent freezing and protects engines and heating/cooling systems from corrosion.
- Cosmetics and Fragrances: Propylene glycol is used as a solvent for cosmetic ingredients and as a carrier for fragrances. It helps in improving the spreadability and absorption of cosmetic products.
- Food and Beverage Additive: Propylene glycol is approved as a food additive by regulatory authorities in many countries. It is used in a variety of food products, including baked goods, dairy products, confections, and beverages, as a thickener, stabilizer, or emulsifier.
- Industrial Applications: Certain unique properties of PG, such as low volatility and high boiling point, make it useful in industrial processes. Propylene glycol is utilized in paints and coatings, detergents, resins, synthetic fibres, and as a heat transfer fluid.
- Pharmaceutical and Personal Care Formulations: PG serves as a pharmaceutical excipient, aiding in the solubilization and stabilization of drugs in various formulations. Additionally, propylene glycol is found in many personal care products such as lotions, creams, and shampoos, providing moisturization and enhancing product stability.
- Solvent and Humectant: Propylene glycol is widely used as a solvent in various industries, including pharmaceuticals, cosmetics, and food. It acts as a carrier for active ingredients and aids in dissolving other substances. Its ability to absorb and retain moisture also makes it a suitable humectant in personal care products.
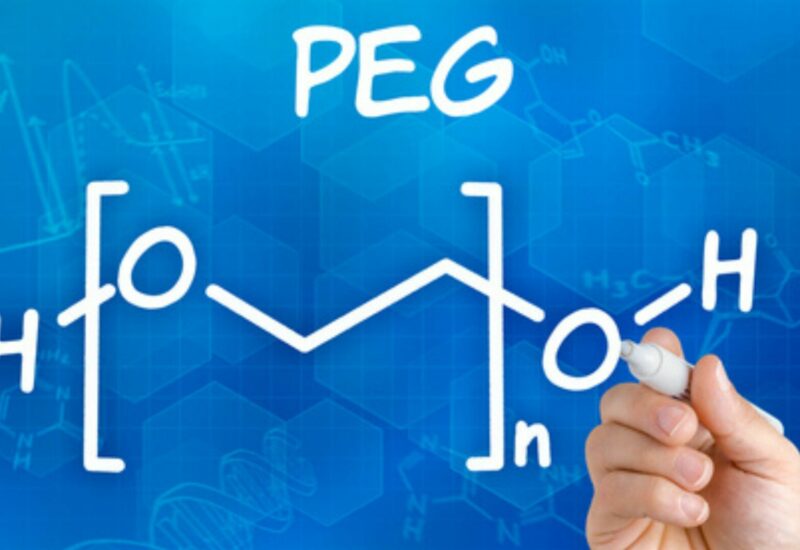
Polyethylene Glycol (PEG) is a polyether compound with the formula (C2H4O)n, where “n” represents the average number of repeating ethylene oxide monomer units. It is a clear, odourless, and viscous liquid or solid, depending on its molecular weight. The production of polyethylene glycol involves the polymerization of ethylene oxide monomers. The process typically begins with ethylene oxide being reacted with water or ethylene glycol under controlled conditions, using a catalyst such as an alkali metal hydroxide or an acid. This polymerization reaction forms long chains of repeating ethylene oxide units, resulting in the formation of PEG with varying molecular weights.
Key applications for PEG include:
- Chemical Industry: PEG serves as a versatile chemical intermediate in various industrial processes. It is used in the synthesis of surfactants, polymers, resins, and plasticizers. PEG is also employed as a freeze-thaw protection agent in certain industries.
- Cosmetics and Personal Care: PEG is widely used in the cosmetics and personal care industry, where it serves as a thickening agent, emollient, or emulsifier in various products such as lotions, creams, shampoos, and soaps. PEG helps in moisturizing the skin, improving product consistency, and enhancing the absorption of active ingredients.
- Food and Beverage: PEG is considered safe for consumption and is used as a food additive. It can act as a solvent, emulsifier, or stabilizer in food products, including baked goods, confectionery, beverages, and dairy products. PEG can improve texture, prevent crystallization, and enhance the shelf life of food items.
- Industrial Applications: PEG finds utility in a range of industrial processes, including being used as a lubricant, dispersant, or additive in textile manufacturing, metalworking, paper production, and polymer processing. PEG is also employed as a humectant and antistatic agent in products like paint, adhesives, and inks.
- Pharmaceuticals: PEG plays a crucial role in many pharmaceutical formulations. It is used as a solvent, excipient, or polymer matrix in drug delivery systems, including oral liquids, creams, ointments, and suppositories. PEG can enhance the solubility and bioavailability of drugs and act as a sustained-release agent.


